
Sicl4 Lewis Structure
Is Sicl4 polar or nonpolar? SiCl4 (silicon tetrachloride) is a nonpolar chemical. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity.
Sf2 polar or nonpolar lenaka
SiCl4 (Silicon tetrachloride) is a non-polar molecule. SiCl4 is non-polar because the four chemical bonds between silicon and chlorine are equally distributed. Keep reading to know more about the question "SiCl4 polar or nonpolar".
SOLVED Determine whether each molecule is polar or nonpolar Drag the
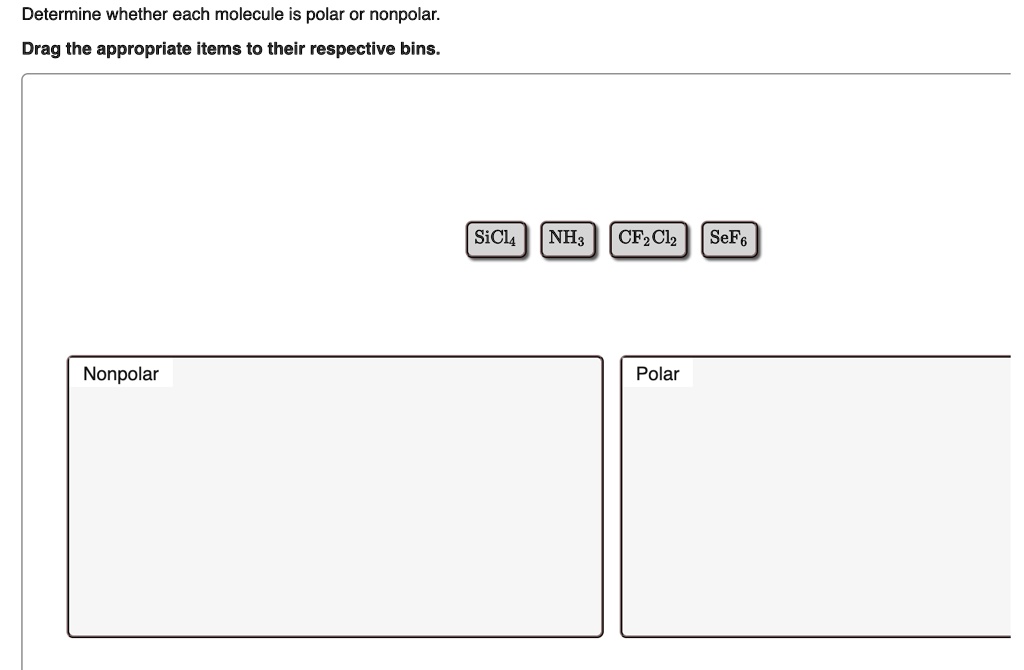
Silicon tetrachloride (SiCl4) is a non-polar molecule. It consists of one silicon (Si) atom and four chlorine (Cl) atoms. The silicon is kept at the central position while all four chlorine atoms occupy surrounding positions, making a perfectly symmetrical tetrahedral molecular shape and geometry.
MakeTheBrainHappy Is SiCl4 Polar or Nonpolar?
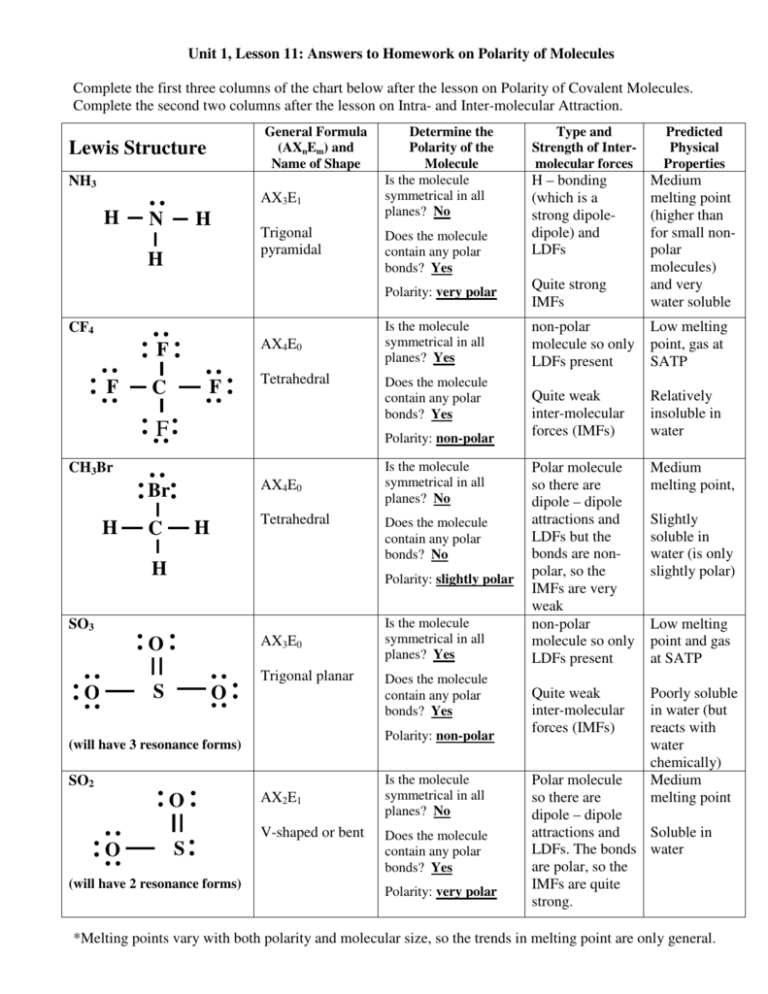
Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. Skip to main content. General Chemistry. Start typing, then use the up and down arrows to select an option from the list.. Polar & Non-Polar Molecules: Crash Course Chemistry #23. CrashCourse. 674. 12:58. 1.5 Polarity. Chad's Prep. 179. 07:23. Molecular.
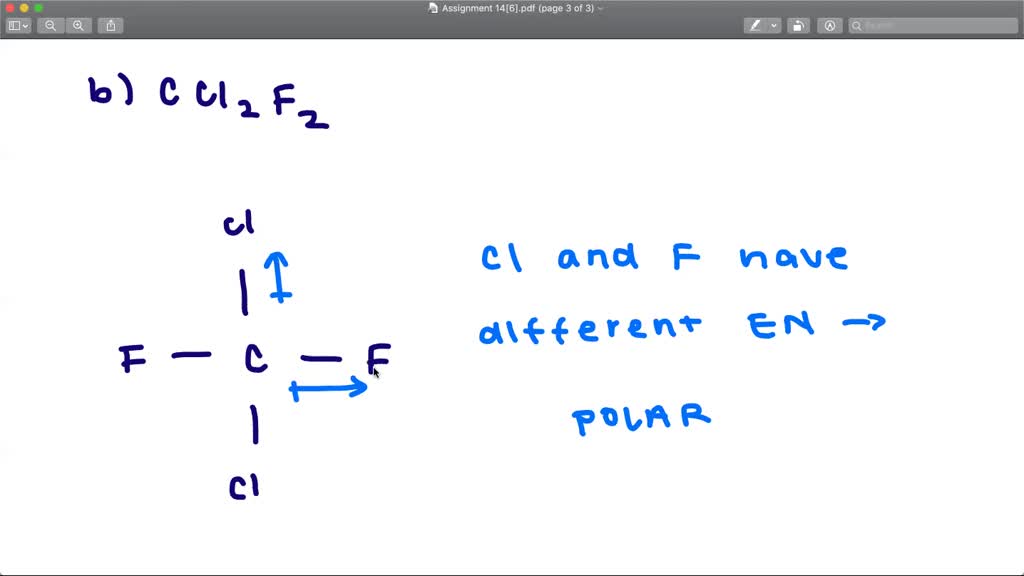
SOLVEDDetermine whether each molecule is polar or nonpolar. a. SiCl4 b
The total valence electron is available for the Silicon tetrachloride (SiCl4) lewis structure is 32. The hybridization of the SiCl4 molecule is Sp 3. The bond angle of SiCl4 is 109.5º. SiCl4 is nonpolar in nature, although, its bonds are polar. The overall formal charge in Silicon tetrachloride is zero.
SiCl4 Polar or Nonpolar Easy Explanation What's Insight
In this article, named as "sicl4 lewis structure", lewis structure, hybridization, geometry, formal charge calculation with some detailed explanations on. SiCl 4 Polar or Nonpolar. SiCl 4 is a molecule with zero dipole moment. But the four Si-Cl bonds are comparatively polar due to the electronegativity difference between silicon and.
Ch4 Polar Or Nonpolar / 9.2h Predicting whether molecules are polar or
Yes, SiF4 is a nonpolar molecule despite four individual Si-F bonds are polar in nature. This is because SiF4 has tetrahedral molecular geometry which means the molecule is symmetrical so that the polarities of the Si-F bonds cancel each other and the net dipole becomes zero. Why is SiF4 a nonpolar molecule? [Detailed Explanation]

Is SiCl4 a Polar or Nonpolar Substance?
SCl4 is a POLAR molecule because the Chlorine (Cl) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire SCl4 molecule polar.

SOLVEDDetermine whether each molecule is polar or nonpolar. a. SiCl4 b
SiCl4 is a chemical that has an asymmetrical tetrahedral form and geometry. This symmetry is that the dipole moments of each polar Si-Cl bond are canceled uniformly across the whole SiCl4 molecule. In the end, SiCl4 is an ionic molecule. It is a compound formed through the reaction between silicon and chlorine.

Is SiCl4 Polar or Nonpolar? Techiescientist
SeCl4 is a POLAR molecule because the Chlorine (Cl) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire SeCl4 molecule polar.

SiCl4 Lewis Structure (Silicon Tetrachloride) YouTube
SiCl4 is a non-polar compound because of its linear and symmetrical shape. The bonds in the molecule are polar because the chlorine atom is more electronegative than the silicon atom but due to linear and opposite directions of both bonds, the dipoles of both bonds in SiCl4 cancel out each other.

SOLVEDClassify each molecule as polar or nonpolar. (a) SiCl4 (b) SiH3
SiCl4 or silicon tetrachloride is a non-polar molecule. It is used in a variety of ways, but mainly in the production of very pure silicon.

r shape Step 1 Write the Lewis structure from the molecular formula
The SiH4 is nonpolar in nature because of its symmetrical shape having four identical Si-H bonds canceling out their dipole moments resulting in net dipole moment zero. Although the Si-H bond is polar because of the difference in their electronegativity, the net dipole moment of the entire molecule is zero making SiH4 a nonpolar compound.

Ch4 Polar Or Nonpolar / Solution Is The Ch4 A Polar Or Non Polar Chemistry
SiCl4 is silicon tetrachloride, which is a non-polar molecule. Silicon tetrachloride is non-polar because the four chemical bonds between silicon and chlorine are equally distributed. The even distribution of polar bonds results in a molecule that does not have distinct poles.
[Solved] what is the lewis structure, electron geometry, molecular
SiCl4 is a NONPOLAR molecule because all the four bonds (Si-Cl bonds) are identical and SiCl4 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SiCl4 lewis structure and its 3D geometry. Why is SiCl4 a Nonpolar molecule? (Explained in 3 Steps)

Is SiCl4 Polar or NonPolar? YouTube
Answer: SiCl4 (Silicon Tetrachloride) is a nonpolar molecule because all of the four chlorine molecules are equally spaced around the central silicon atom in a tetrahedral structure. As the electronegativity difference between chlorine (3.16) and silicon (1.90) is quite high, the bonds within the molecule are polar covalent.