Ethanol Polar Or Nonpolar homework How do I figure out the relative
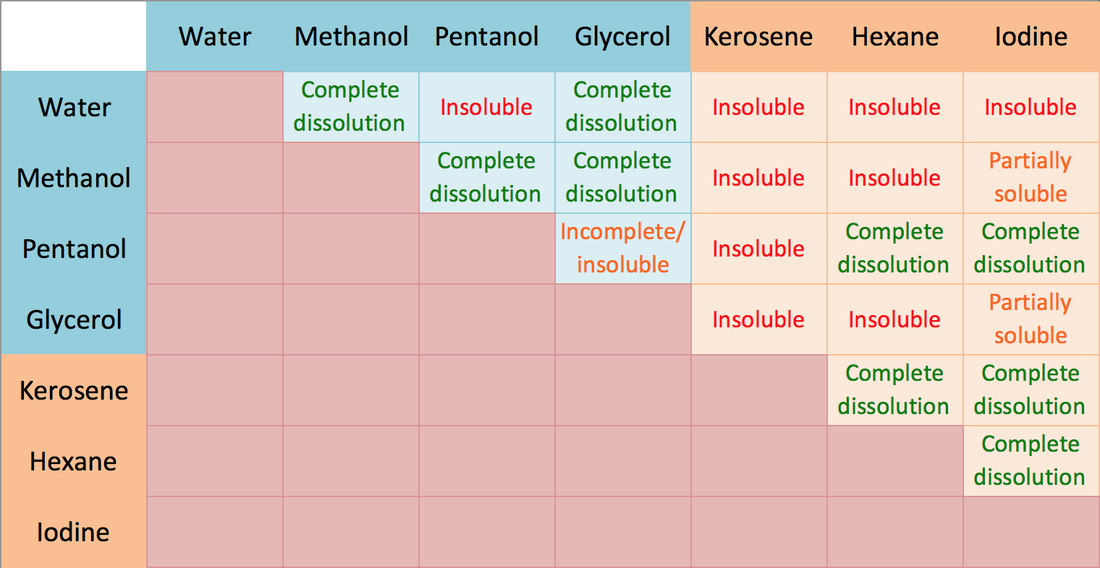
Polar solutes are typically quite soluble in polar solvents (e.g., ethanol in water), and nonpolar solutes generally dissolve well in nonpolar solvents (e.g., grease in gasoline). Conversely, polar solutes will have low solubilities in nonpolar solvents (e.g., NaCl in CCl 4 ), and solubilities will be low for nonpolar solutes in polar solvents (e.g., oil in vinegar).

Ethanol Polar or Nonpolar EmilykruwDennis
C2H5OH or Ethanol can simply be called or termed alcohol and it is an organic chemical compound. The compound can also be represented as CH3-CH2-OH. Ethanol is a colorless liquid with a distinct odor and a pungent taste. It has flammable properties; when burnt, the compound gives a blue color flame. Here are some ways in which ethanol is prepared:
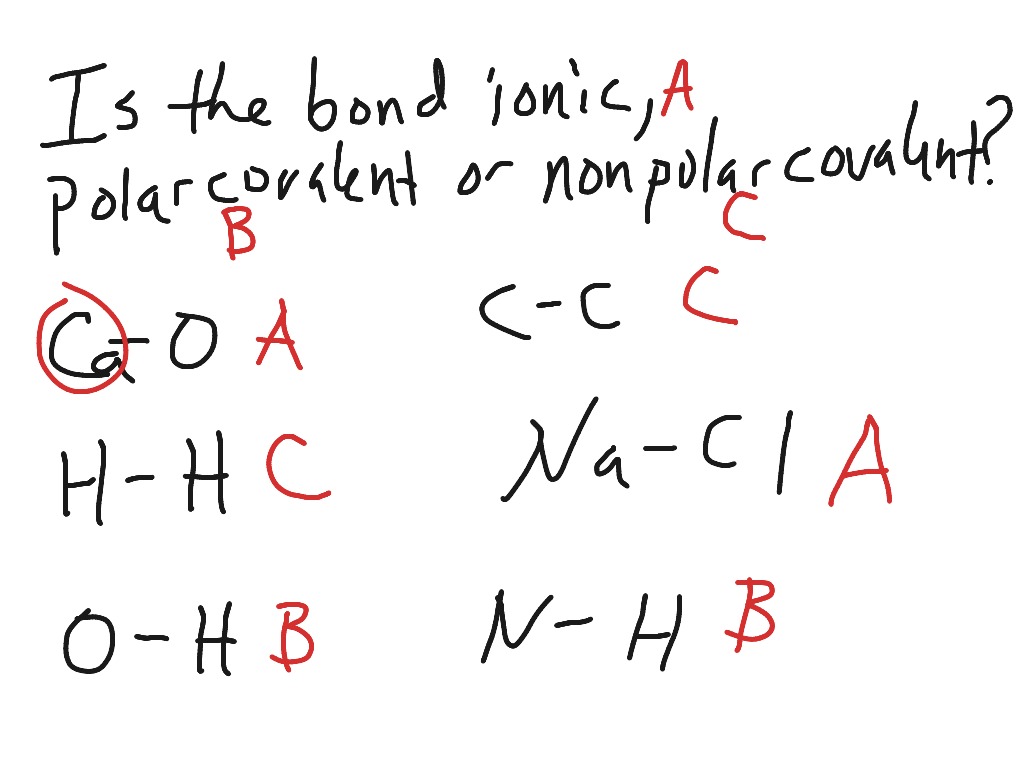
Ethanol Polar Or Nonpolar homework How do I figure out the relative
© 2023 Google LLC Learn to determine if C2H5OH (Ethanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.
Solved H2O hexane ethanol acetone H20 Polar or nonpolar?
ethanol, a member of a class of organic compounds that are given the general name alcohol s; its molecular formula is C 2 H 5 OH. Ethanol is an important industrial chemical; it is used as a solvent, in the synthesis of other organic chemicals, and as an additive to automotive gasoline (forming a mixture known as a gasohol ).
Ethanol Polar Or Nonpolar homework How do I figure out the relative
C2H5OH is a chemical formula for Ethanol, an organic chemical compound. It is a flammable, volatile and colourless liquid having a characteristic odour. When it comes to Ethanol and other alcohols, many of our students and readers have doubts regarding their polarity.
ethanol png 20 free Cliparts Download images on Clipground 2023
Ethanol | CH3CH2OH or C2H6O | CID 702 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
The Ethanol Molecule Ethanol
Ethanol is a volatile, flammable, colorless liquid with a characteristic wine -like odor and pungent taste. [13] [14] It is a psychoactive recreational drug, and the active ingredient in alcoholic drinks . Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration.
News & Info Applied Separations Replace Ethanol & Polar Solvents in
Ethanol is polar in nature because of the presence of the hydroxyl group (-OH) attached to the carbon end. Due to the difference between the electronegativity of oxygen and the hydrogen atom, the hydroxyl group becomes polar. As a result, the molecule of ethanol gives non zero dipole moment and becomes a polar molecule.
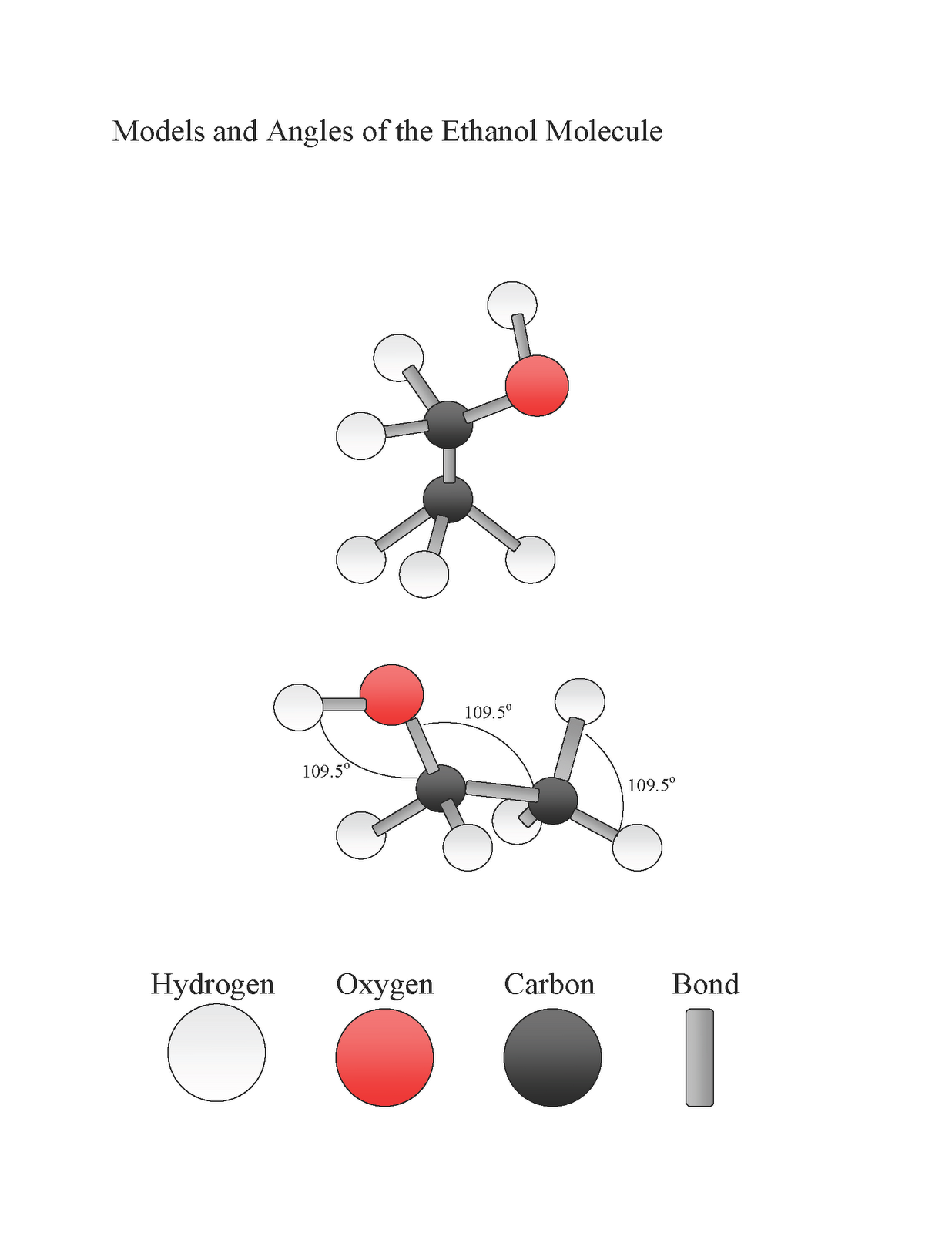
MakeTheBrainHappy Is Ethanol Polar or Nonpolar?
The OH groups of alcohol molecules make hydrogen bonding possible. Recall that physical properties are determined to a large extent by the type of intermolecular forces. Table 14.3.1 14.3. 1 lists the molar masses and the boiling points of some common compounds. The table shows that substances with similar molar masses can have quite different.

draw the structure of ethanol molecule greenbrierapartmentsannarbor
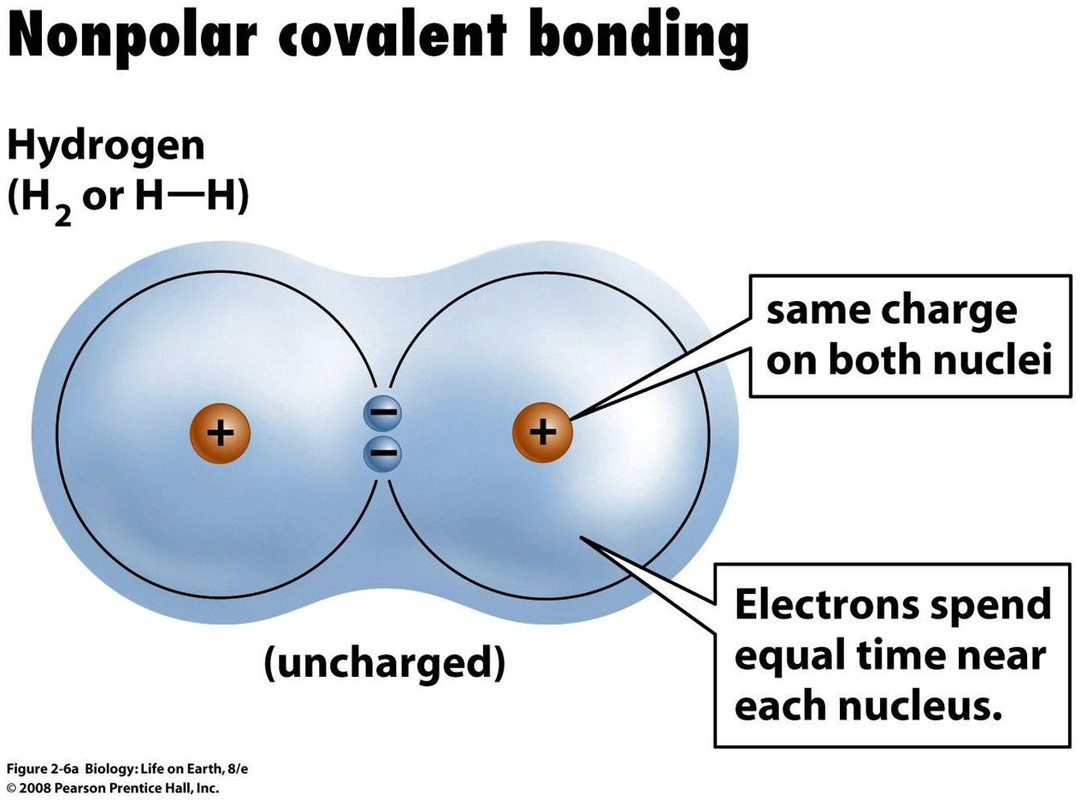
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Solved Predict which bond is the most polar in ethanol, How
An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group. Alcohols are common in nature.

Ethanol Polar or Nonpolar KeelnReilly
Methanol (CH 3 OH) and ethanol (CH 3 CH 2 OH) are the first two members of the homologous series of alcohols. Alcohols with one to four carbon atoms are frequently called by common names,. Like the H-O-H bond in water, the R-O-H bond is bent, and alcohol molecules are polar. This relationship is particularly apparent in small.
Ethanol Polar Or Nonpolar homework How do I figure out the relative
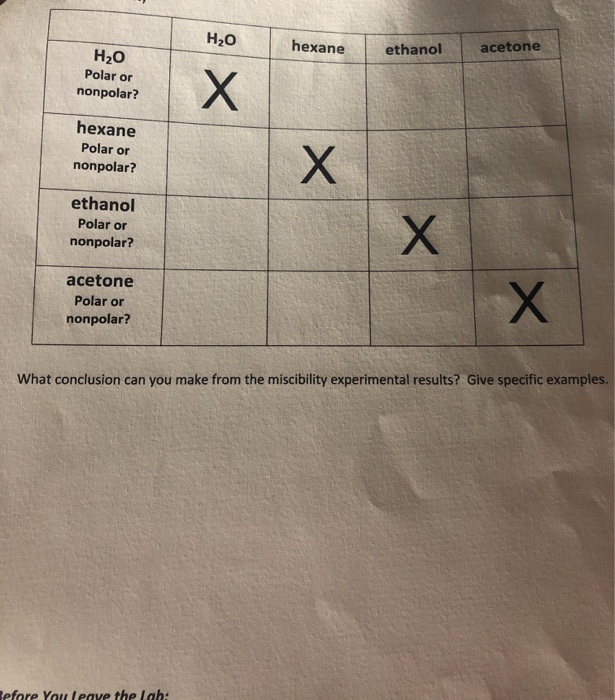
True. Ethanol does on a molecule by molecule basis have stronger intermolecular forces between itself and water than methanol and water. and more soluble in water than methanol. False. Yet, my chemistry textbook says that both ethanol and methanol are miscible in water, but the more the carbon chain increases, the less miscible it becomes. True.
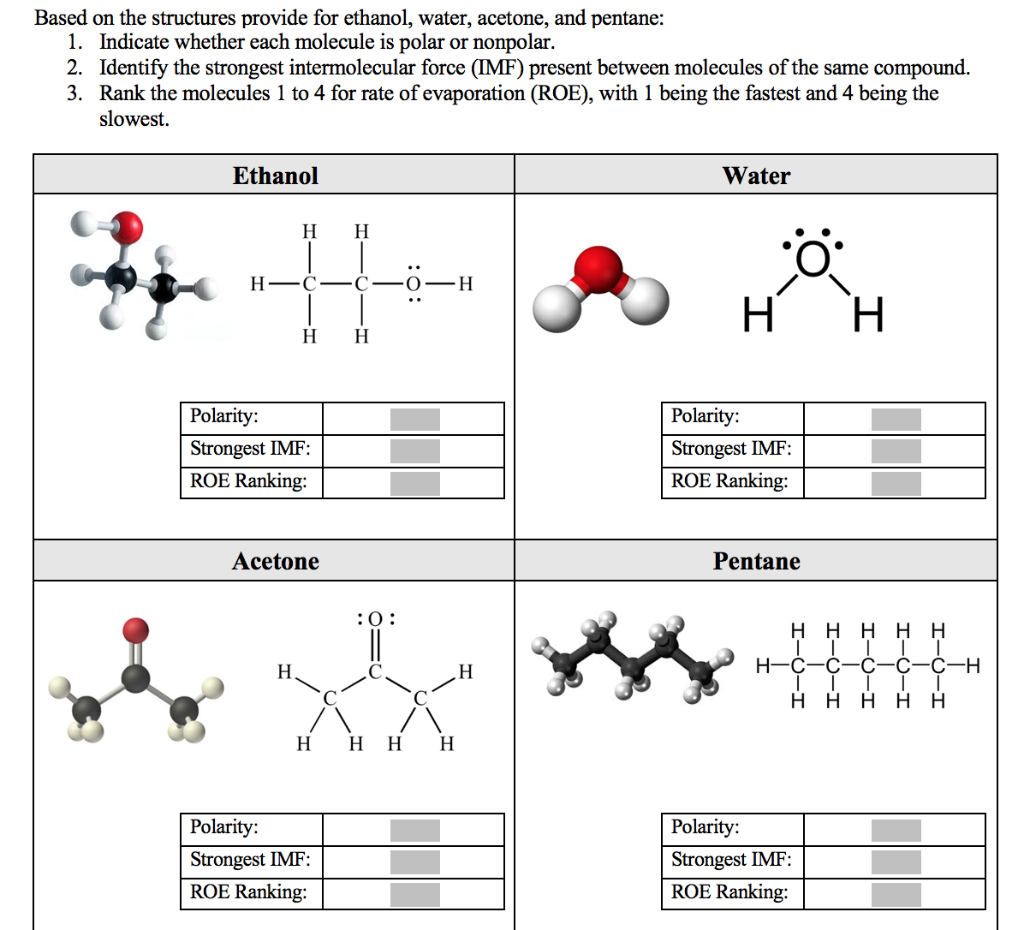
Solved Based on the structures provide for ethanol, water,
About Transcript Organic compounds tend to dissolve well in solvents that have similar properties to themselves. This principle is often referred to as "like dissolves like," which means that polar molecules will generally dissolve well in polar solvents and non-polar molecules will generally dissolve in non-polar solvents. Questions Tips & Thanks

Spectrum of phenol in isooctane and ethanol. Polar solvents such as
Like the H-O-H bond in water, the R-O-H bond is bent, and alcohol molecules are polar. This relationship is particularly apparent in small molecules and reflected in the physical and chemical properties of alcohols with low molar mass.. hydrocarbon than ethanol is. The molar mass of 1-hexanol is greater than that of 1-butanol. Key.

Answered Ethanol Dimethyl ether 7th attempt Part… bartleby
Ethanol (C2H5OH) is a POLAR molecule because the Oxygen (O) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire C2H5OH molecule polar.