Resonance Structures Practice Master Organic Chemistry
Resonance Structures Organic Chemistry Practice Quiz Not sure if you've mastered resonance structures? Looking for extra practice? Resonance is a critical topic in your organic chemistry curriculum. After watching the Resonance Structures Video Series test your knowledge with this short quiz below.

️Resonance Worksheet Organic Chemistry Free Download Gmbar.co
Resonance structures are required throughout organic chemistry. You'll learn how to draw resonance early in orgo 1, and be tested on resonance intermediates in advanced orgo 2 mechanisms. To help you take away the guesswork I've put together a brand new series taking you through the basics, starting with the question: What IS Resonance, all the.

Resonance in Organic Chemistry — Organic Chemistry Tutor Organic Chemistry Tutor, Forensic
The following problems are meant to be useful study tools for students involved in most undergraduate organic chemistry courses. The problems have been color-coded to indicate whether they are: 1. Generally useful, 2. Most likely to be useful to students in year long, rather than survey courses, 3.

6.2. Resonance Organic Chemistry 1 An open textbook
1: Basic Concepts in Chemical Bonding and Organic Molecules
draw all reasonable resonance structures for the following species jimmorrisonpaintingpawnstars
About this unit Let's review how to keep track of electrons using formal charges, oxidation states, oxidation-reduction reactions, and resonance structures. We will also go over the principles of acid-base chemistry. Counting electrons Learn Comparing formal charges to oxidation states Formal charge on carbon Formal charge on nitrogen
organic chemistry Archives Organic Chemistry Made Easy by
Organic Chemistry Date_____ Period _____. Draw all of the reasonable resonance structures for each of the following molecules. a. f. k. p. H b. g. l. q. c. h. m. r. d. i. n. s. e. j. o. t. H2N O CN 2N O NH2 O N CN O NH2 O CN H2N O H2N NH2 O N CN H2N O O O HN HN CN O O OH. Answers to Even More Resonance Practice Problems a. b. c. H2N O H2N O.

How to Study the Resonance Effect in Organic Chemistry 6 Steps
The equivalent ressonance structures seem like the same but there are non equivalent ressonance strutures that occur when the delocalization of electrons is between qualitativity different bonds (they are different because they bond different atoms for instance a nitrogen and a carbon and two carbons) ( 6 votes) Upvote Show more.

Resonance of Ketones and Esters (Carbonyls) Organic Chemistry Resonance Practice YouTube
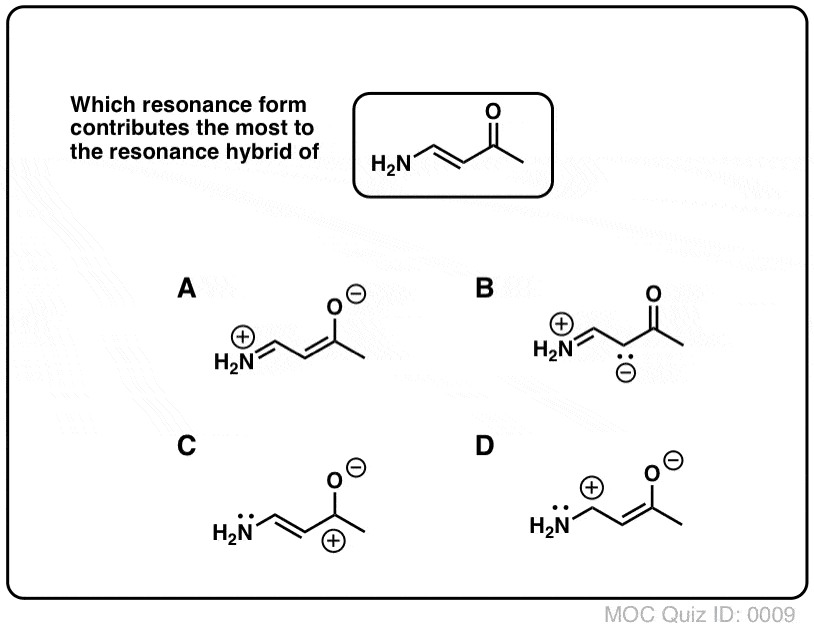
Resonance Structures Practice These questions cover all aspects of resonance in organic chemistry, from identifying resonance forms, understanding partial charges, ranking the relative importance of resonance forms, and drawing curved arrows to interconvert resonance forms. Identifying Resonance Forms Quiz#:5 Click to Flip Solution Video-A1-5
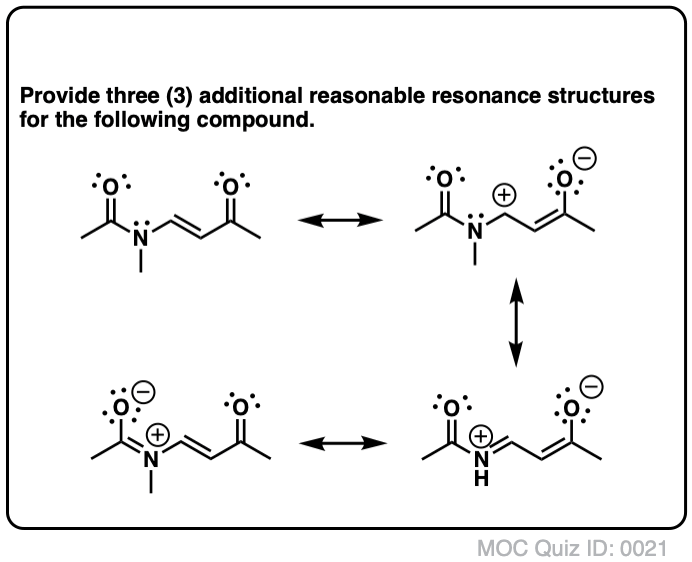
draw the resonance structure of a peptide bond cheaperboschcrevicetool
Organic Chemistry (Morsch et al.) 2: Polar Covalent Bonds; Acids and Bases 2.6: Drawing Resonance Forms Expand/collapse global location
What is resonance? [7 rules to master it & free guide] Organic chemistry help
Practice Sets Organic Chemistry II Table of Contents • Online Organic Chemistry II, Chem 360,. resonance anions more stable than anions without resonance Test 1 PS# 2: Acid Base P ractice Set. Organic(Chemistry(2((Jasperse( ( Test(1,(Alcohol(Chemistry(
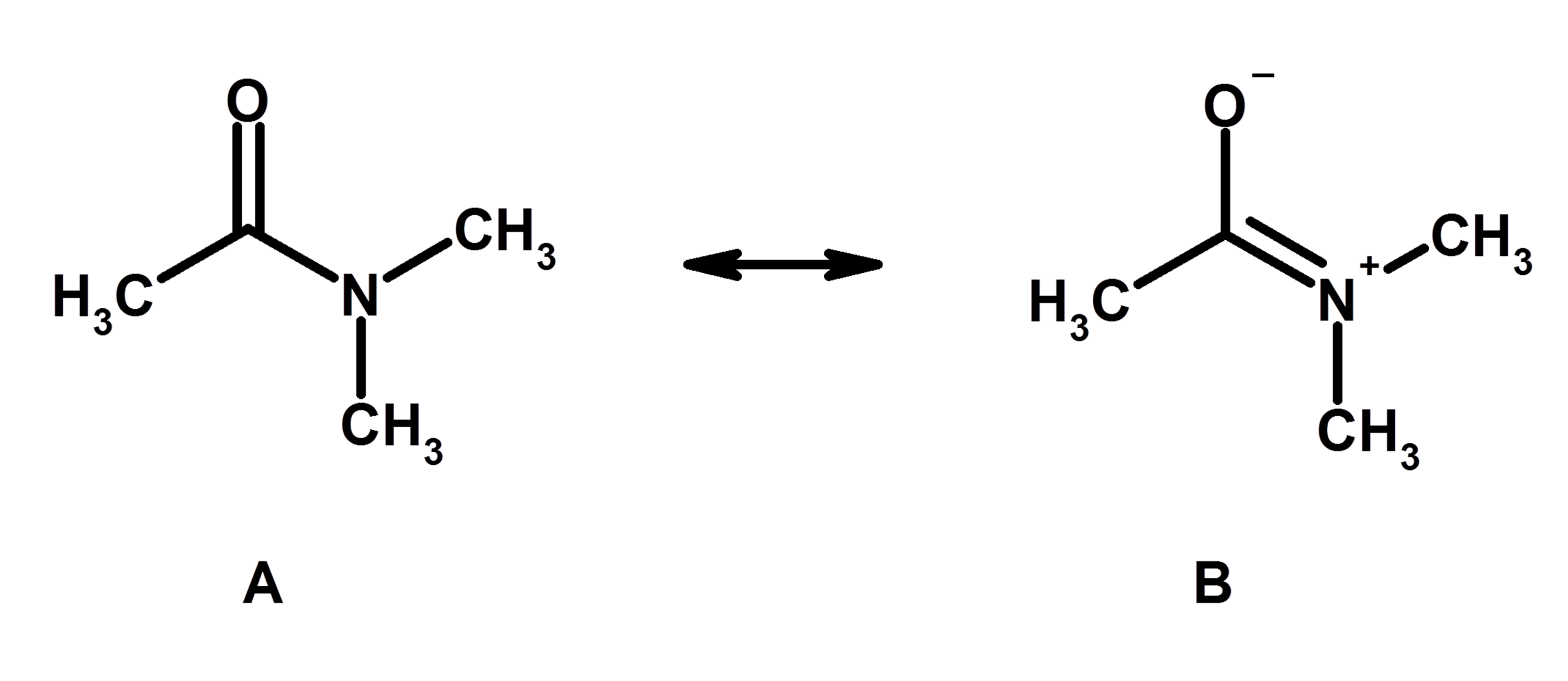
1.3 Resonance Structures Organic Chemistry I
1.3 Resonance Structures - Organic Chemistry I 1.3 Resonance Structures In cases in which more than one reasonable (plausible) Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be either equivalent or non-equivalent. Equivalent Resonance Structures
draw a second resonance structure for the following radical doodleArtDrawingSchool
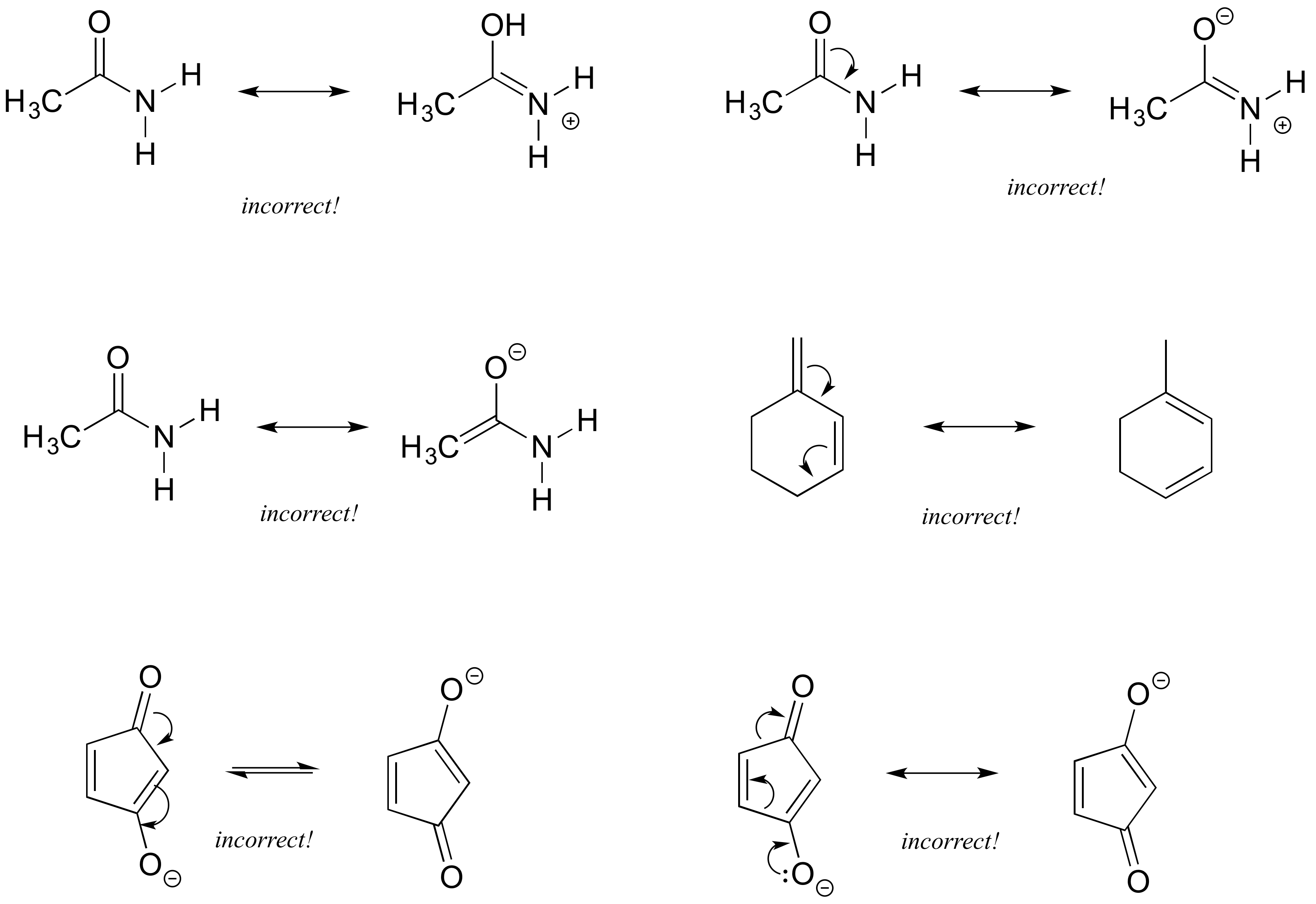
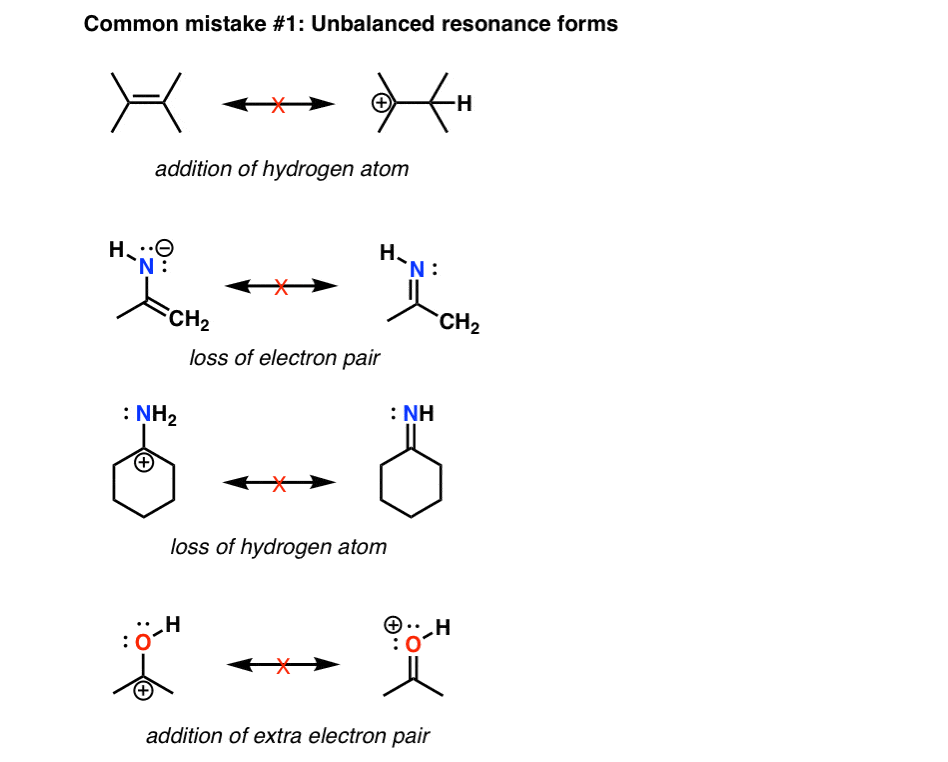
As we've seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Rule #1: Minimize charges Rule #2: Full octets are favored Rule #3: How stable are the negative charges? Rule #4: How stable are the positive charges?
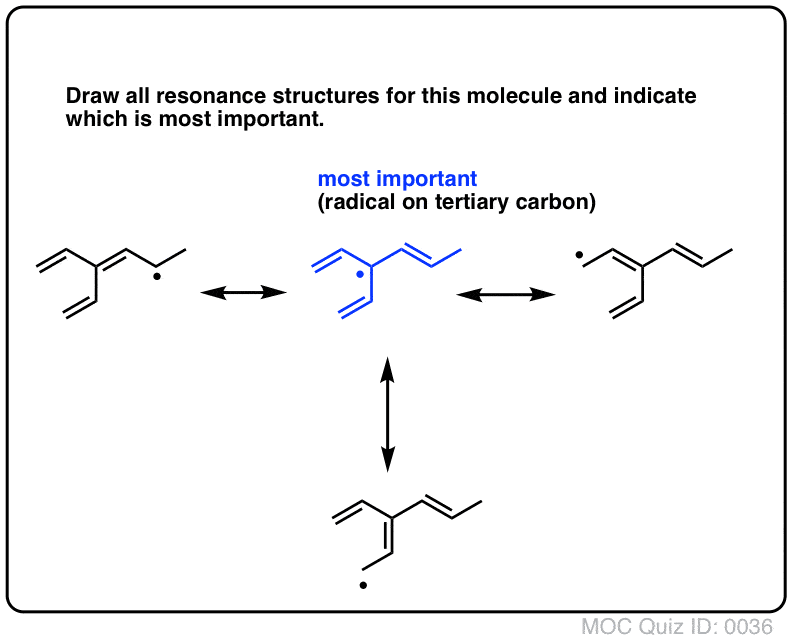
Resonance Structures Practice Master Organic Chemistry
The Resonance stabilization effect (also known as the resonance effect ), as briefly mentioned in Section 1.3, is one of the fundamental concepts of Organic Chemistry and has broad applications. The discussion of the resonance effect heavily relies on the understanding of resonance structures.

Resonance Structure Practice Worksheet
Organic Chemistry Molecular Representations Resonance Structures Practice Problems In the previous post, we talked about the resonance structures and the main rules applied to them. In summary, there are two must-follow rules when drawing resonance structures: 1) Do not exceed the octet on 2nd-row elements. 2) Do not break single bonds

Lewis Structures in Organic Chemistry Chemistry Steps
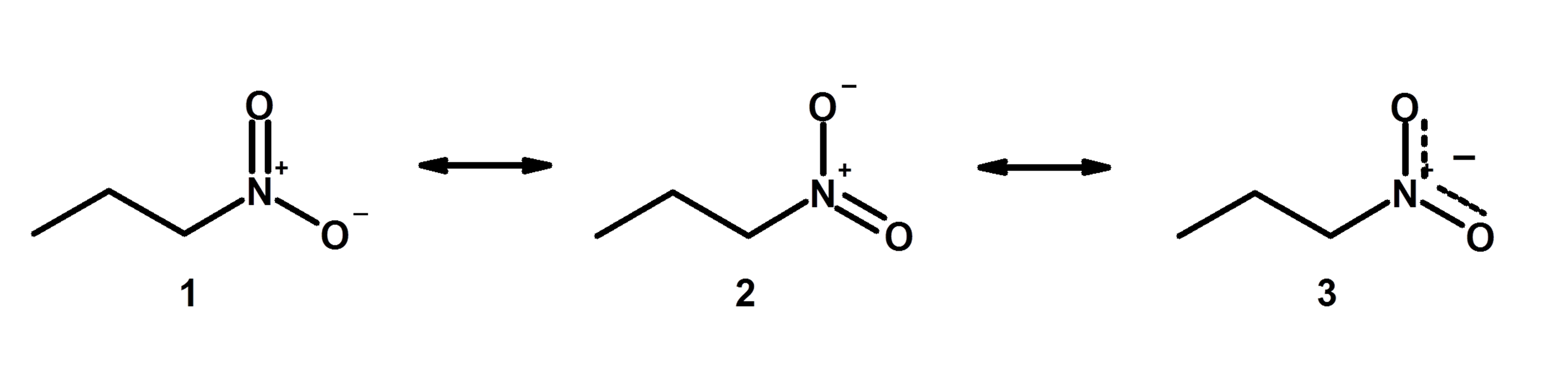
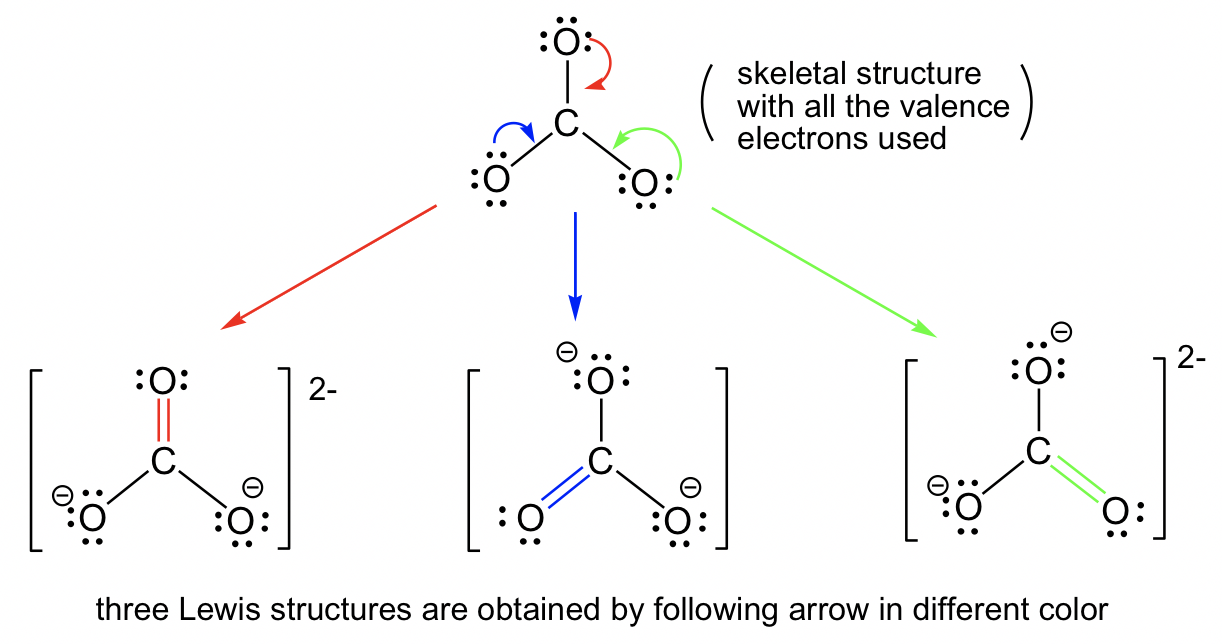
Answers to Practice Questions Chapter 1. Non-equivalent Resonance Structures. Resonance structures can also be non-equivalent. For the example of OCN -, there are three non-equivalent resonance structures, depending on how the multiple bonds are formed in Step 6 of the Lewis structure drawing procedure.. Figure 1.3e Three non-equivalent resonance structure contributors of OCN-

Practice drawing resonance structures organic chemistry ideas in 2022 DRAWING 99
Choose 1 answer: All of the bonds in CO A 3 A 2 − are identical in length and strength. A. All of the bonds in CO A 3 A 2 − are identical in length and strength. The bonds in CCl A 4 are more polar than the bonds in CH A 4 . B. The bonds in CCl A 4 are more polar than the bonds in CH A 4 . Both of the bonds in BeH A 2 have a bond order of 1 .