Electron Shell Sodium Electron Configuration Bohr Model PNG, Clipart, Area, Atom, Atomic Number
Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Sodium has one valence electron, so giving it up would result in the same electron configuration as neon. Chlorine has seven valence electrons, so if it takes one it will have eight (an octet)..
Electron Configuration for Sodium (Na, and Na+ ion)
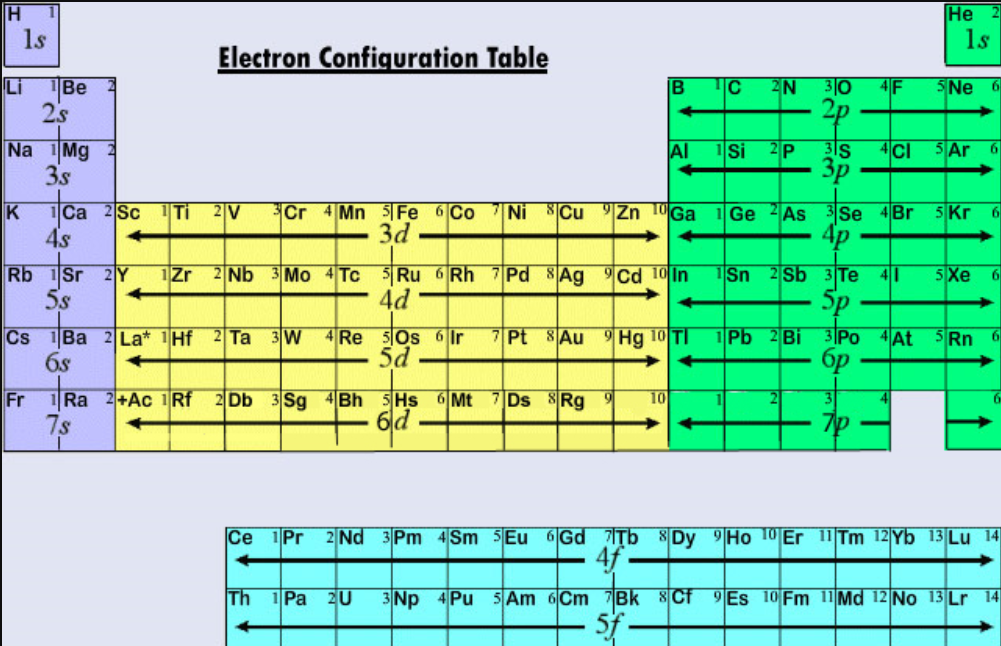
There are four simple steps to find out the valence electrons for sodium atom which are: Step 1: Find the Atomic Number To find out the atomic number of sodium, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of sodium is 11.

How Many Valence Electrons Does Sodium(Na) Have?
Method 2: From the Electron Configuration. If you want to find the valence electrons of sodium from its electron configuration, then you should know its electron configuration first. Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle. Aufbau principle: The.

Sodium Na (Element 11) of Periodic Table NewtonDesk
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
Sodium Valence Electrons Sodium Valency (Na) with Dot Diagram
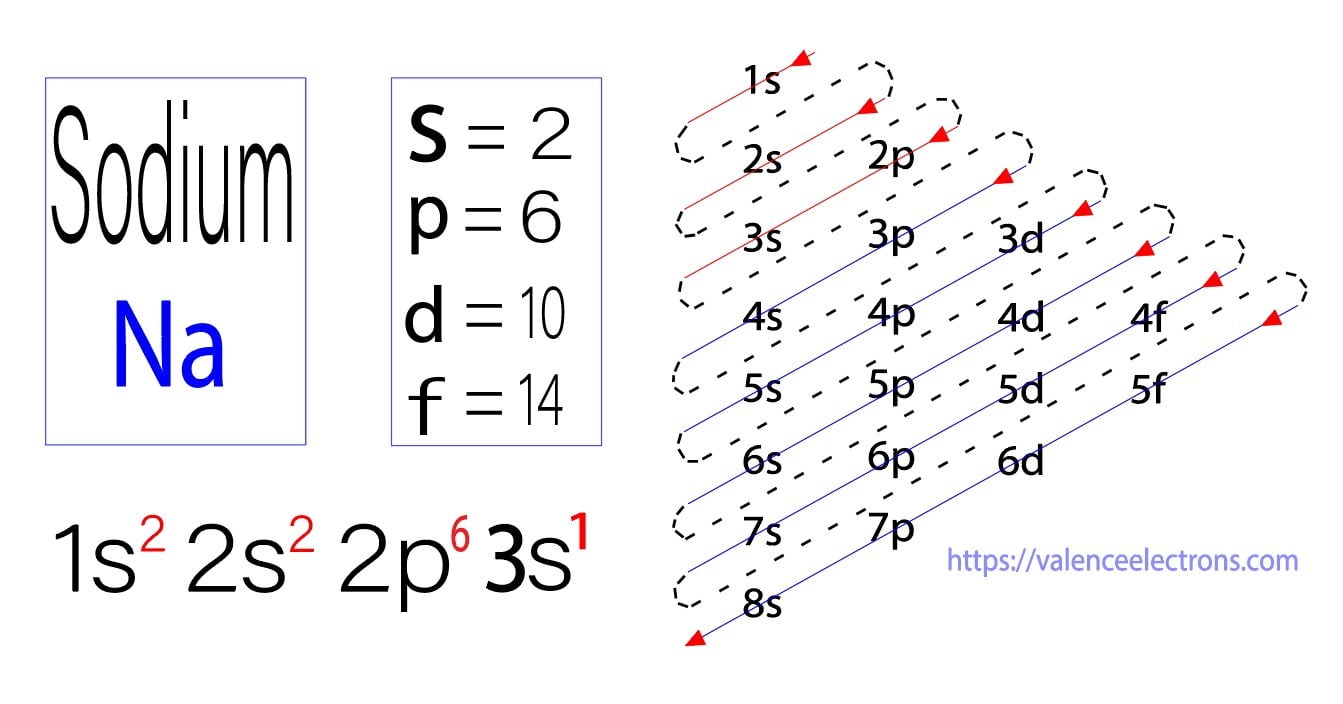
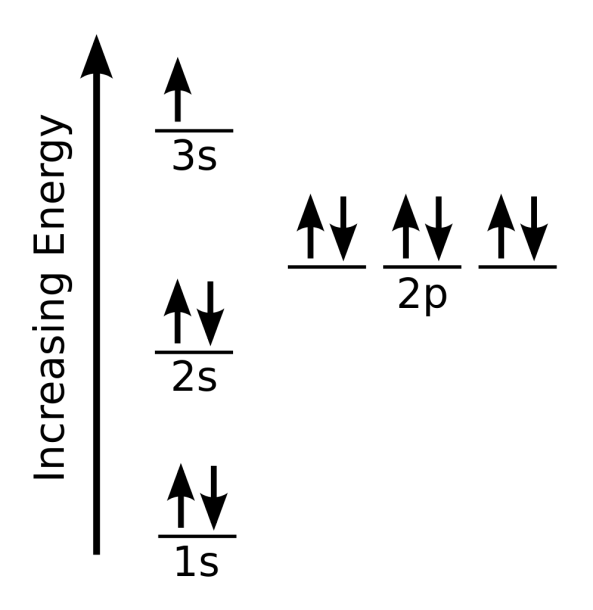
And you have one more electron to worry about. And so that electron would go into a 3S orbital. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level.

How Many Valence Electrons Does Sodium(Na) Have?
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions.

Sodium(Na) electron configuration and orbital diagram
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.

How Many Valence Electrons Does Sodium(Na) Have?Number of valence electrons in sodium
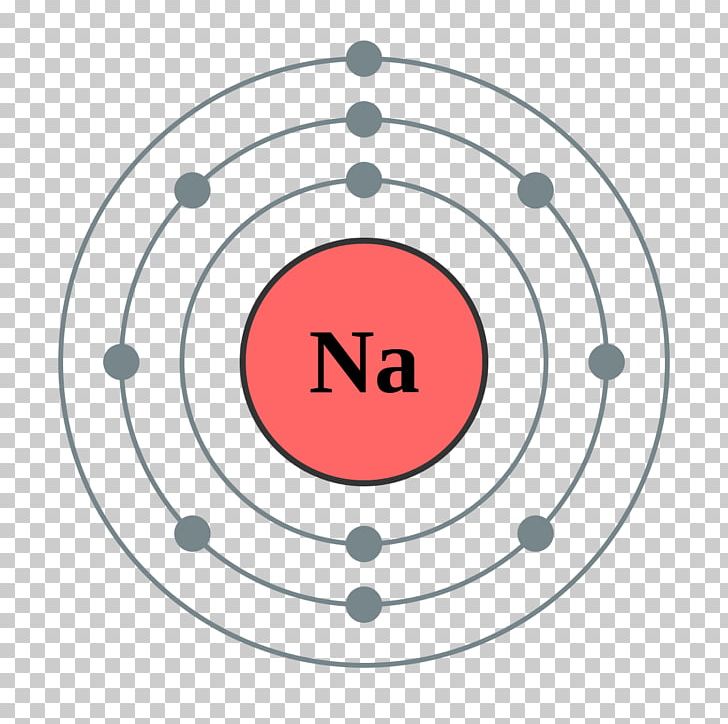
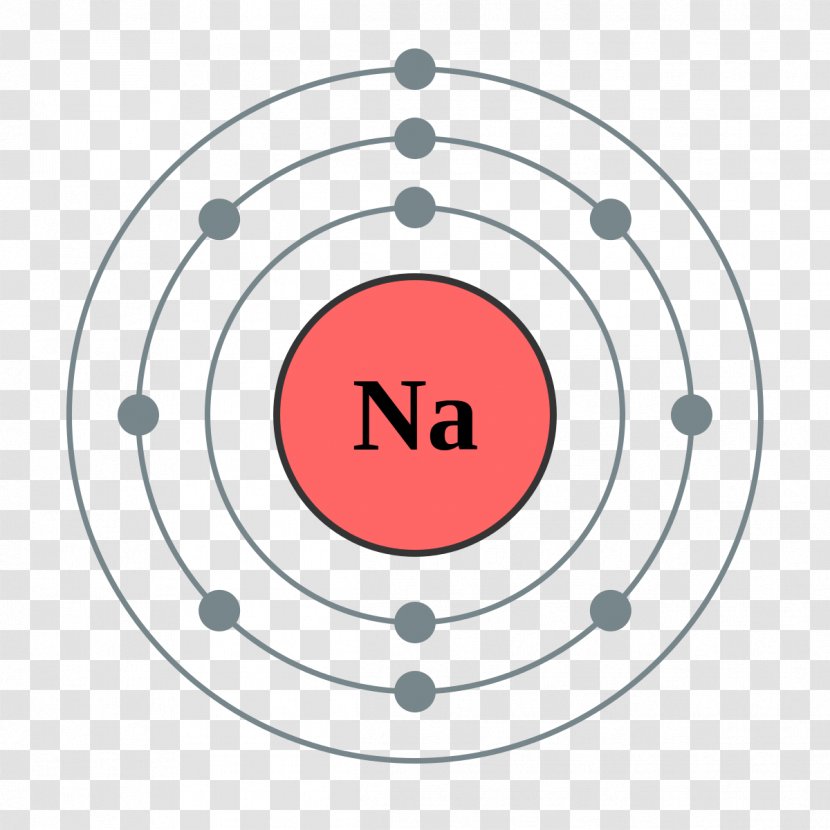
Sodium has one valence electron. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. The third shell, which is the outermost and the valence shell, has only one electron. Valence electrons influence chemical reactivity. How Valence Electrons Influence Chemical Reactions
Electron Shell Sodium Configuration Bohr Model Valance Transparent PNG
March 23, 2023 by Jay Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.

Bohr Diagram Of Sodium
Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals beryllium and magnesium each have two, and the halogens fluorine and.
Valency of Sodium How many valence electrons does Sodium (Na) have?
As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: how likely it is to form bonds, and with which other elements. Electron shells and the Bohr model
Sodium Orbital Diagram
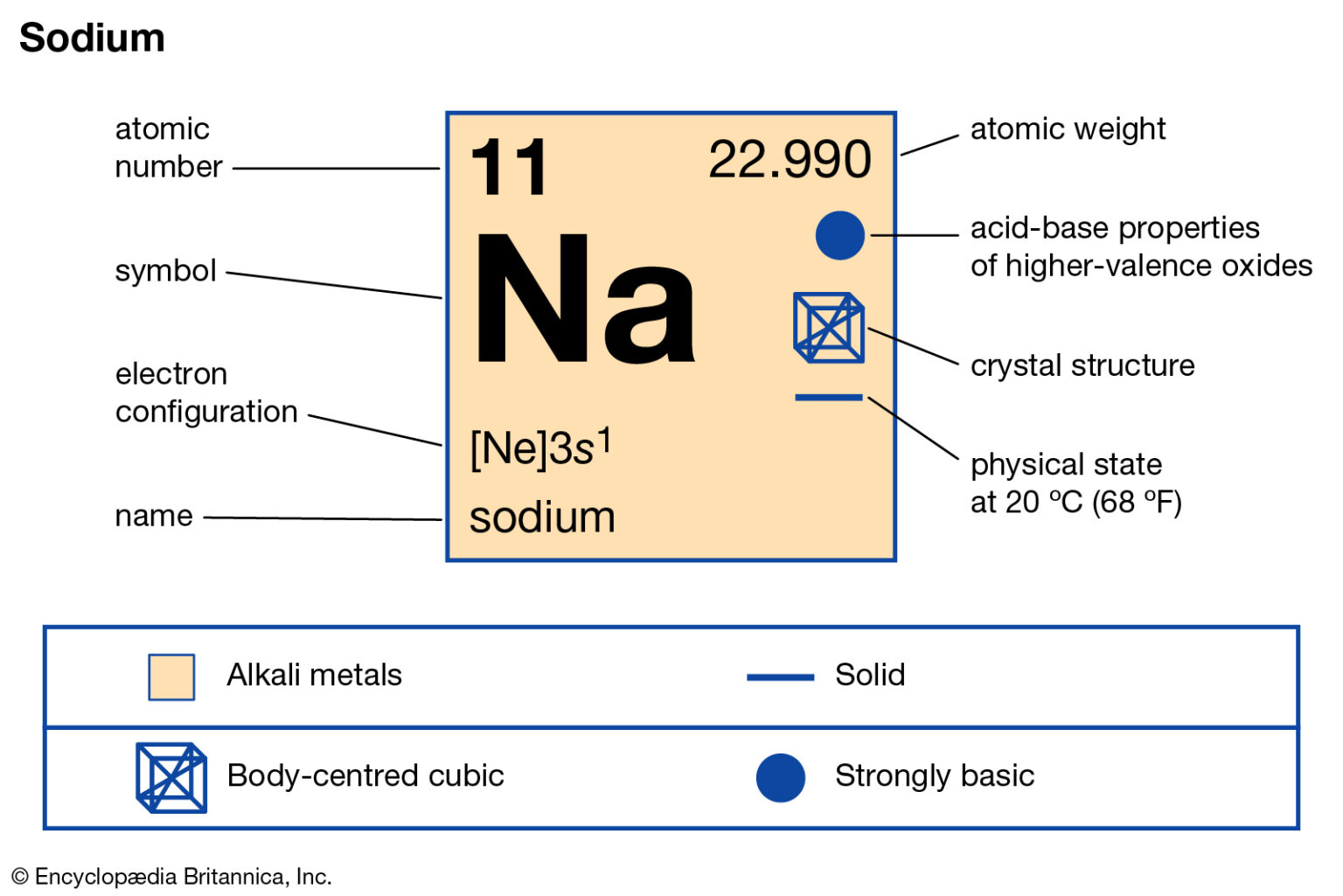
Sodium is a classified alkali metal element. In this article, I have discussed in detail how to easily write the complete electron configuration of sodium. What is the electron configuration for sodium? The total number of electrons in sodium is eleven. These electrons are arranged according to specific rules in different orbitals.

Electron Arrangement of Sodium
What are the valence electrons of sodium? Sodium is an element of group-1. The valence electron is the total number of electrons in the last orbit (shell). The total number of electrons in the last shell after the electron configuration of sodium is called the valence electrons of sodium.

Sodium Orbital diagram, Electron configuration, and Valence electrons
Anne Marie Helmenstine, Ph.D. Updated on November 04, 2019 You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.

How to find Valency? What are valence electrons? Teachoo (2023)
The electron configuration of sodium is [ Ne] 3s 1. Second, find highest energy level in electron configuration. In the above electron configuration, the highest energy level (3) is marked with green color. The 3 rd energy level contains 3s subshell and it has 1 electron. So sodium has a total of 1 valence electron.

Valency of Sodium How many valence electrons does Sodium (Na) have?
Sodium, like all the group 1 alkali metals, has one valence electron. Explanation: Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons.